6 Which of the Following Atoms Has the Smallest Radius
Atomic radius can be defined as a property of elements in the periodic table which is the measure of the distance between the nuclei of two identical atoms bonded together. Which of the following has the smallest atomic radius.
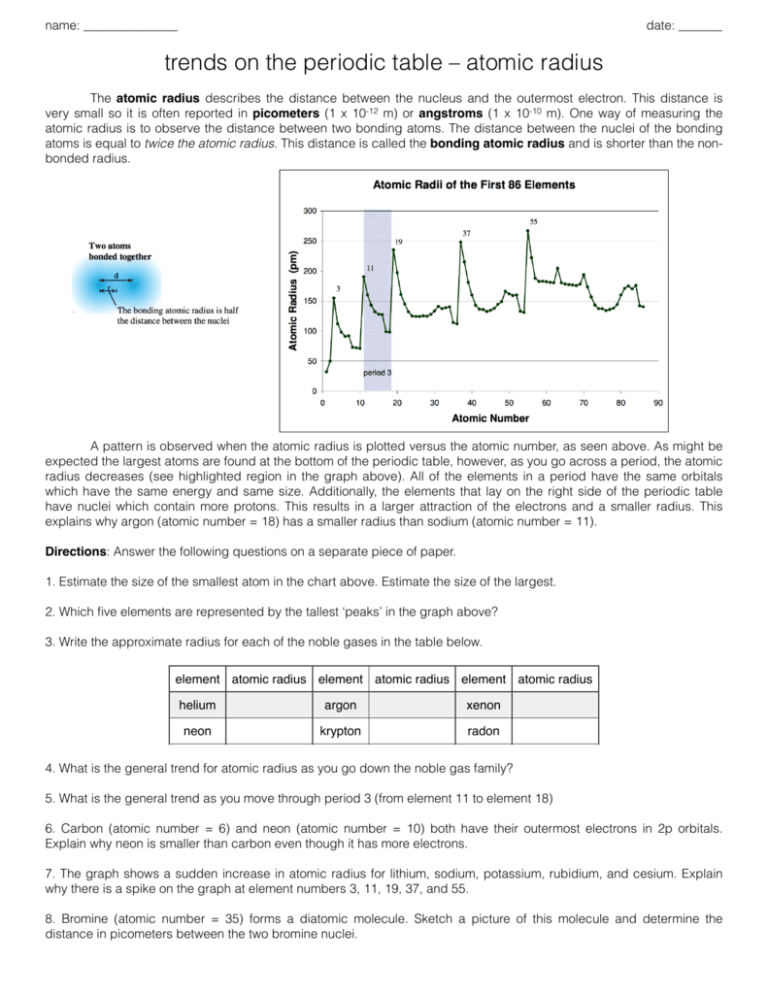
Atomic Radius Practice Problems
From the given elements potassium has the largest atomic radius.
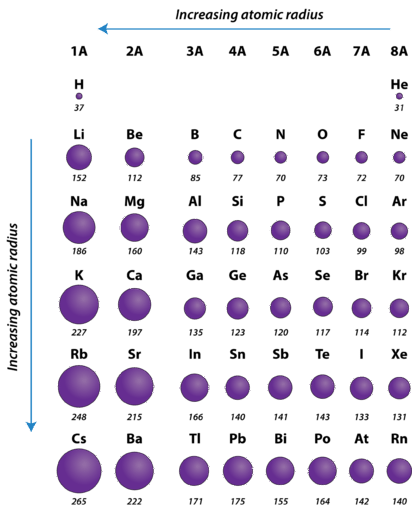
. Helium has the smallest atomic radius. Li Al Be Ba O. When rubidium ions are heated to a high temperature two lines are observed in its line spectrum at.
A photon of light produced by a surgical laser has an energy of 30271019 J. D A andor B. Which of the following atoms has the smallest radius.
Which of the following atoms or ions has the smallest radius. So there is not a complete answer for this question. Which of the following atoms has the smallest atomic radius.
The atomic radius decreases as we move from left to right along the period. Which one of the following has the smallest atomic radius. Thus Br has the smallest atomic radius.
In a periodic table atomic radius decreases as you move across a period from left to right. 32- Which of the following substances will NOT obey the octet rule for at least one atom. Which of the following ionsatoms has the smallest radius.
Na Get the answers you need now. View solution View more. Rank the following atoms in order of increasing size ie smallest to largest.
Thus helium is the smallest element and francium is the largest. Correct option is C When we move from left to right in the Periodic Table atomic radius decreases. Element Z is larger than Element X.
C Element Z and X are probably in the same group. Heated lithium atoms emit photons of light with an energy of 29611019 J. The atomic radius of lithium is smaller than beryllium.
E B andor C. Compared to the atomic radius of a carbon atom the atomic radius of a silicon atom is larger because of an increase in. CLASSES AND TRENDING CHAPTER.
On moving left to right. Based on this you could say. The element that has the smallest atomic radius is Fluorine.
Ca2 D K OE Ar OF. Moreover the radius of alkali metals is metallic radius and that of halogens is covalent radius and metallic radius covalent radius. On moving left to right in the periodic table the atomic size decreases.
The element which has the smallest atomic mass is Hydrogen H which has a proton and an electron. View solution Which one of the following is the smallest cation. A Element Z is further to the left side of the periodic table.
Also in the periodic table atomic. Ocmgrbcs or arpalnak -barbie H smallest S Si Na Rb largest. Rank these elements in terms of increasing atomic radius.
And The atomic radius increases down the group. The order of increasing atomic radius of all atoms above is. Materials Devices and Simple.
B Element X is closer to the top of the periodic table. Elements Z and X are compared. Atoms Chemical Kinetics Moving Charges and Magnetism Microbes in Human Welfare Semiconductor Electronics.
Ar Br Cl Sn Se Pb. Smallest covalent radius By rusd registration packet April 18 2022 sound leaking from headphones. While in a group atomic radius increases from up to down.

The Periodic Table Of The Elements Trends In Atomic Radius Electronegativity Ionizati Ionization Energy Periodic Table Of The Elements Chemistry Worksheets
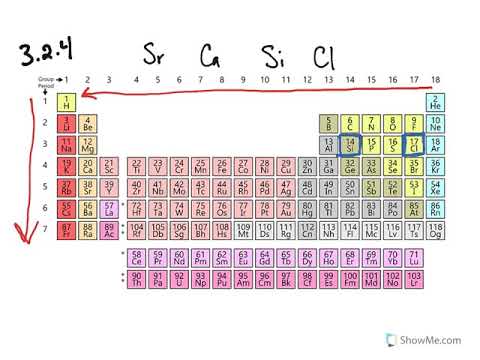
3 2 Trends In Size Problems Chemistry Libretexts
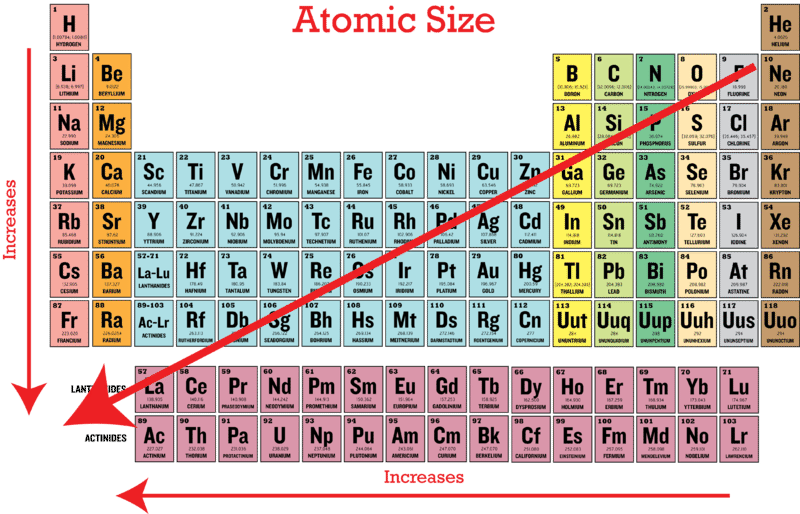
Comments
Post a Comment